
BMR (Batch Manufacturing Record)
G. Batch Production Record Review (6.7) Written procedures should be established and followed for the review and approval of batch production and laboratory control records, including packaging.

Batch Manufacturing Record (BMR) Pharmaceutical Manufacturing M A N O X B L O G
What should be included in a batch manufacturing record? A Batch Manufacturing Record (BMR) is a document that contains step-by-step documentation of the entire manufacturing process involved in producing a product batch, including the expected batch yields and labeling requirements.
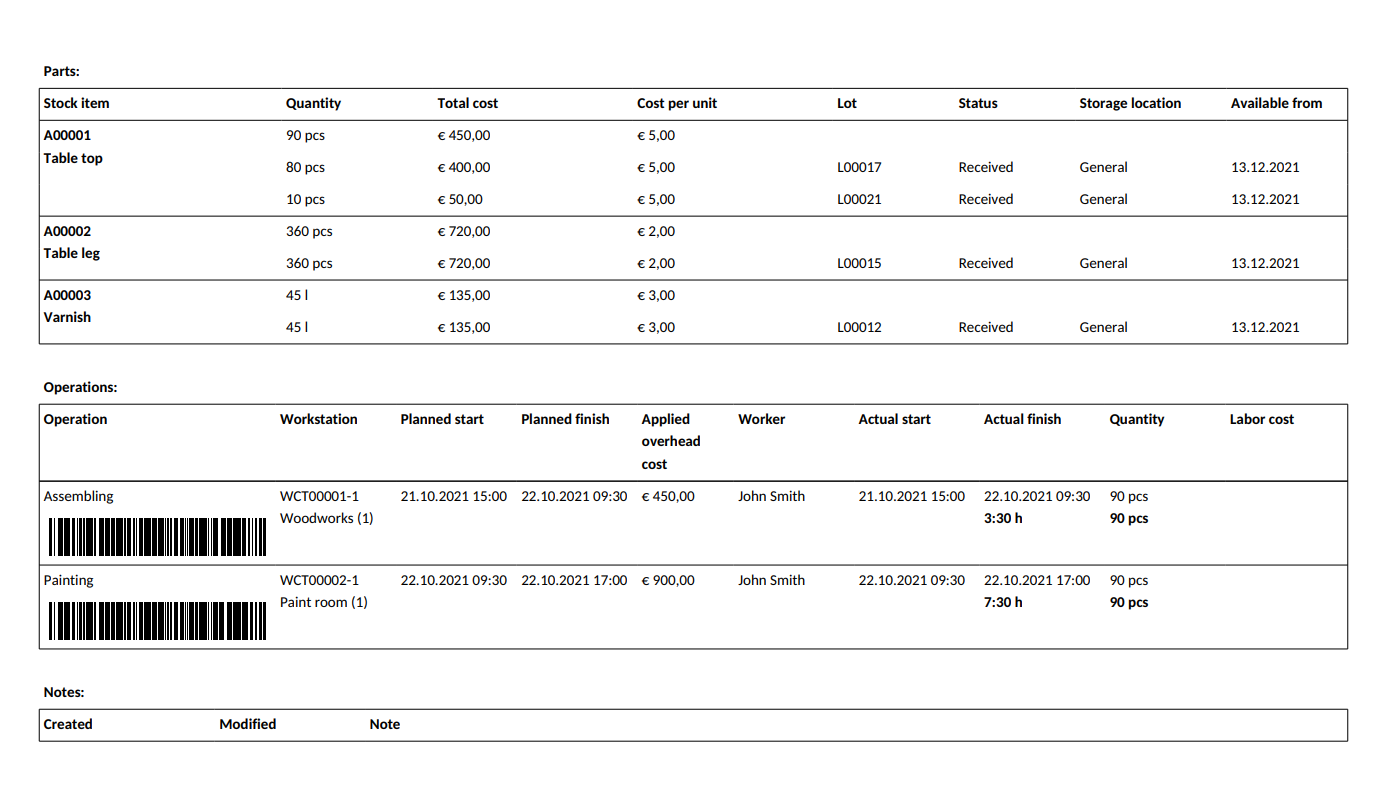
How to Keep Perfect Batch Records? MRPeasy
A batch manufacturing record, or BMR, is a document containing the details of the manufacture of each product batch, across the whole manufacturing process. As there are many stages in the manufacturing process, each step must be recorded as proof, from obtaining the raw materials through to the final stage of packaging ready for sale.
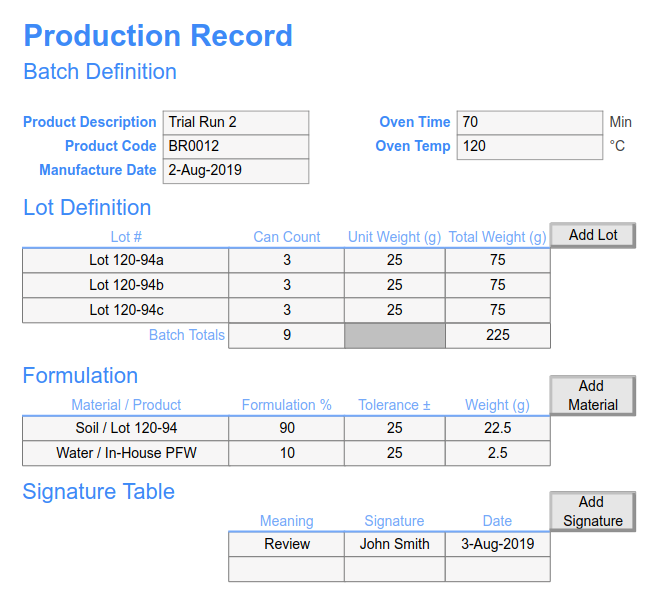
Batch Records SciCord, LLC
A batch manufacturing record or BMR is a very important document & it contains all the manufacturing history of the batch so always put the real-time & actual data in the BMR during each step & don't manipulate the data because the manipulated or fake data will create difficulties during the investigation of any batch if required.

Check List of Batch Record Production And Manufacturing Industrial Processes
Batch manufacturing record is a written document from the batch that is prepared during the pharmaceutical manufacturing process. It contains actual data of the batch manufacturing and whole manufacturing process step by step. There are several stages of the pharmaceutical product manufacturing process.
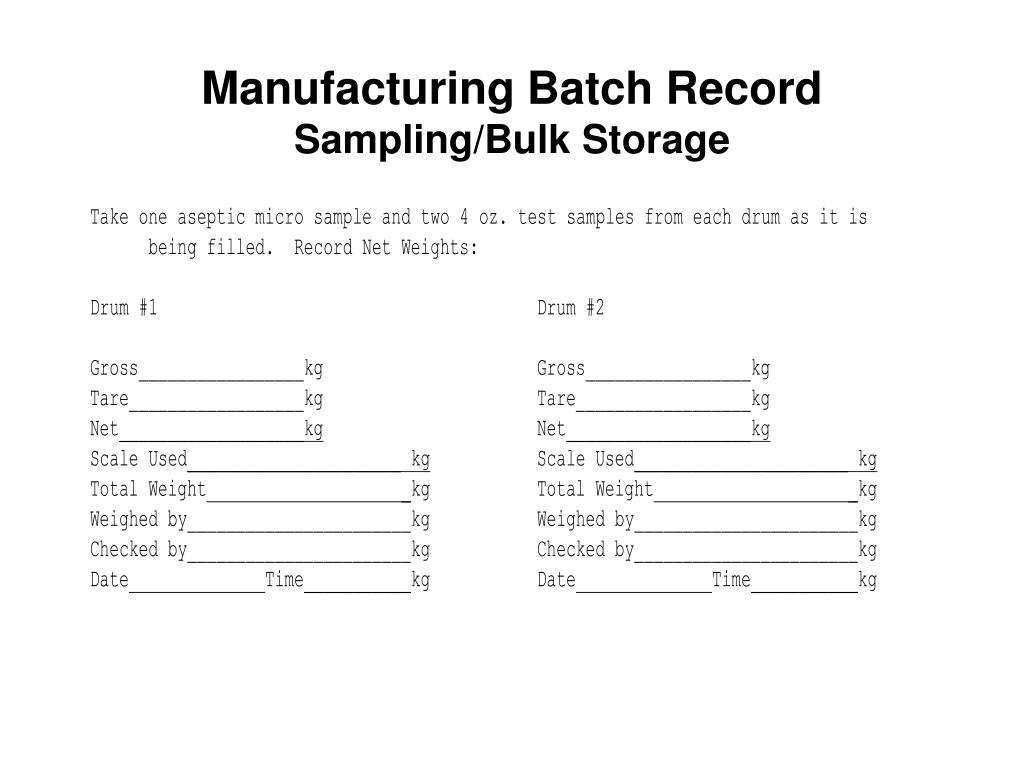
PPT MANUFACTURING DOCUMENTS PowerPoint Presentation, free download ID728573
July 25, 2023 | Business Tips Behind the Scenes: Unraveling the Structure and Components of Manufacturing Batch Records Producing a batch record to verify quality and compliance is an important administrative task for manufacturers, especially in highly regulated industries.

Batch records are a critical part of maintaining Good Manufacturing Practices. This excel
A Batch Production Record (BPR) is a document that provides a detailed record of the production process for a specific batch of a pharmaceutical product. It includes information on the materials and equipment used, the production steps taken, and the testing and analysis performed throughout the production process.

Top Pdf Batch Manufacturing Record Sample Format most complete Forres
A batch manufacturing record is a written and validated narration of the end-to-end manufacturing process. It includes the records of production, instructions on how to manufacture a product, quality control measures, the procedures involved, inspection reports, and the report on the outcome.
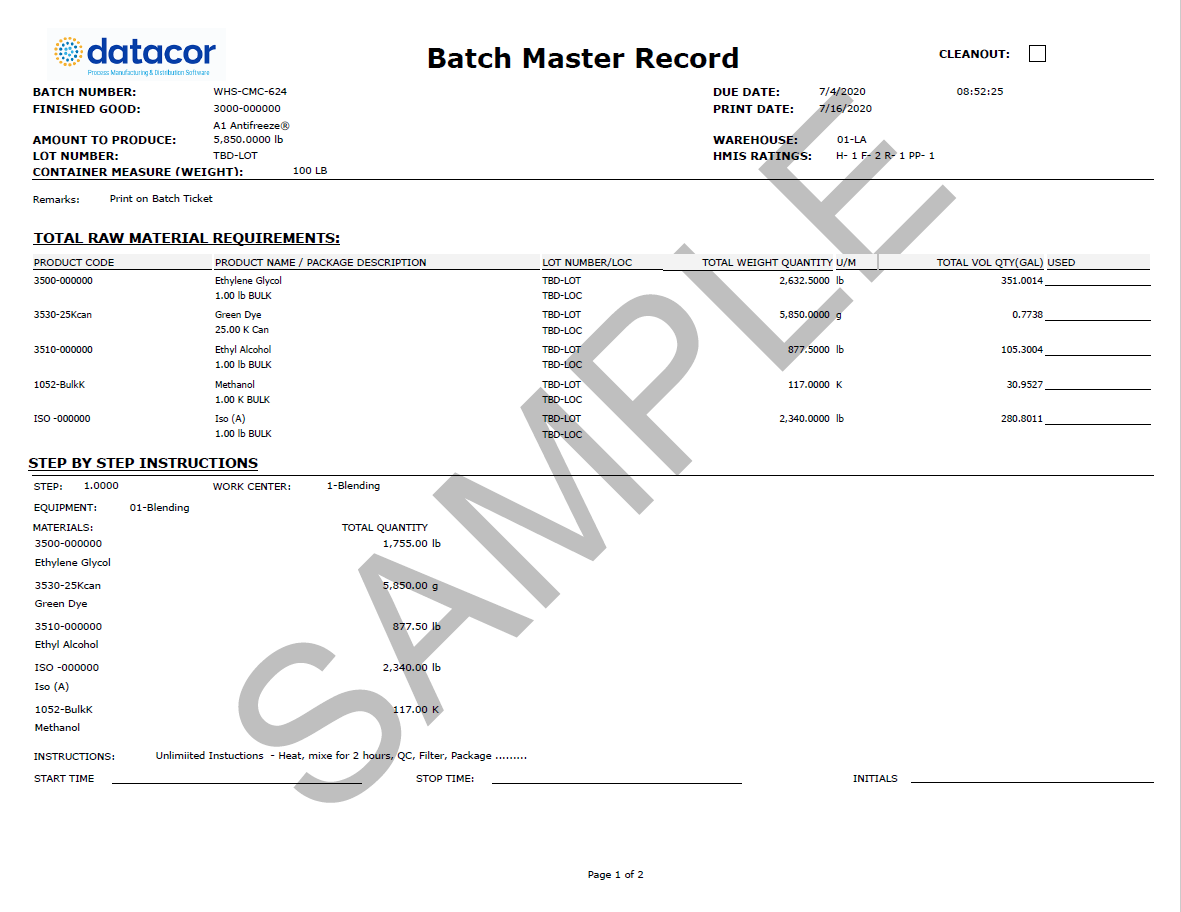
How to Prepare a Batch Manufacturing Record & Free Template (2023)
A batch record is a comprehensive set of documents that outlines all aspects of the Batch manufacturing records (BMRs) Batch production records (BPRs) Master production records (MPRs) This is the most comprehensive type of batch record. It includes all the data associated with the manufacturing process, from raw materials to

How to Prepare a Batch Manufacturing Record & Free Template (2022)
A batch manufacturing record (BMR) is an important document for chemical and process manufacturers: It tells users how to produce a batch of a given product, then records the entire production process, from start to finish.

Sample of Batch Manufacturing Record (BMR) Atorvastatin PDF Download M A N O X B L O G
A master batch record (MBR), also known as a master production record (MPR) is a document that contains the approved ingredients, formulation, and instructions guiding the production of a pharmaceutical product. The document enables manufacturers to follow the necessary regulatory guidelines when manufacturing a product.

PHARMACEUTICAL BATCH MANUFACTURING RECORD Sample Download M A N O X B L O G
In pharmaceutical manufacturing, MES most often means using electronic batch records (EBR) — a digital record that provides proof of a product's batch record with details on process steps, equipment information, materials and supplies. The MES system is centered in manufacturing operations with connections to other foundational business and.
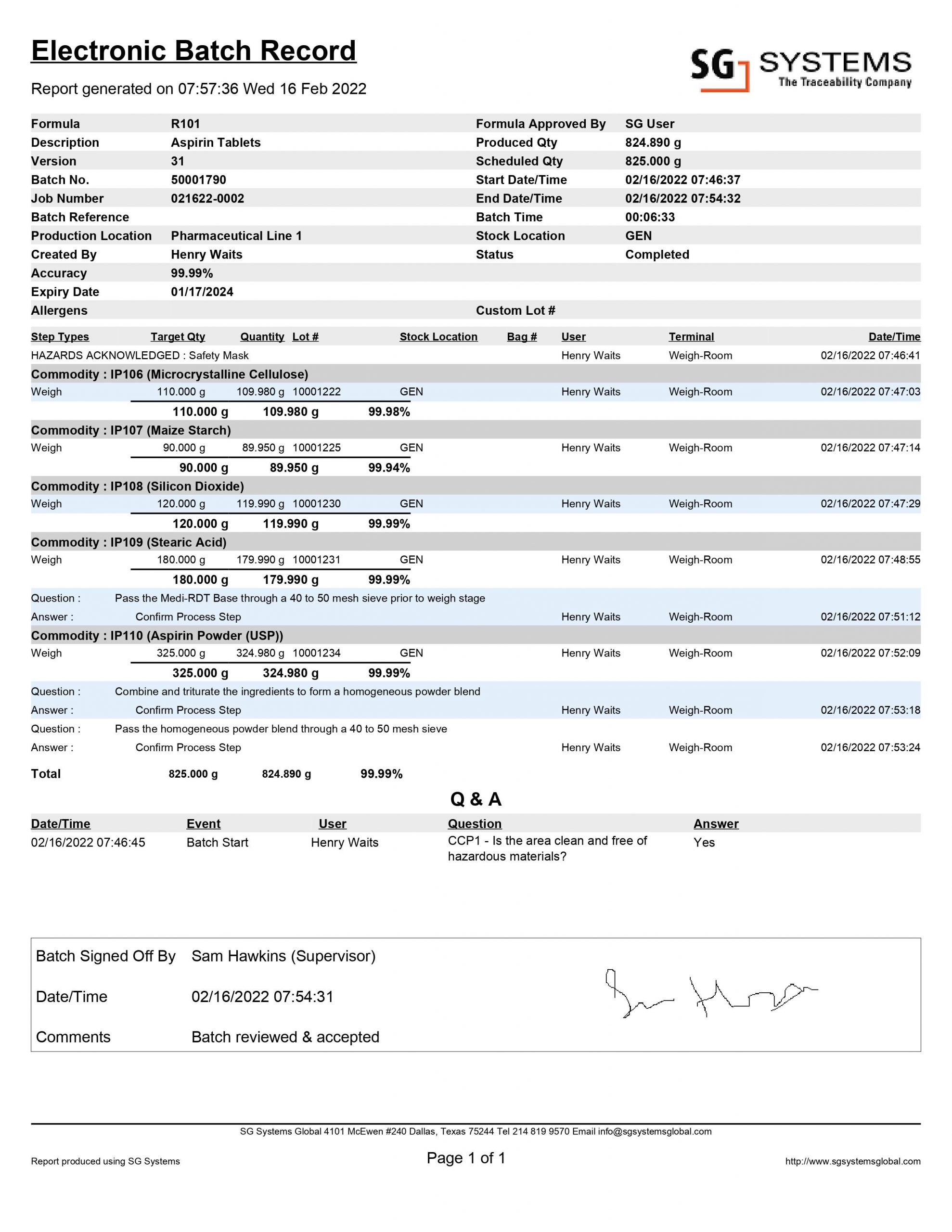
Electronic Batch Record (EBR) 21 CFR Part 11 Digital Compliance
The batch manufacturing record (BMR) is a document containing the instructions that must be followed when manufacturing medication. It includes information like product name, weight and count of each component in the medication, a list of all processes and procedures to follow, and the expected yield of each batch.
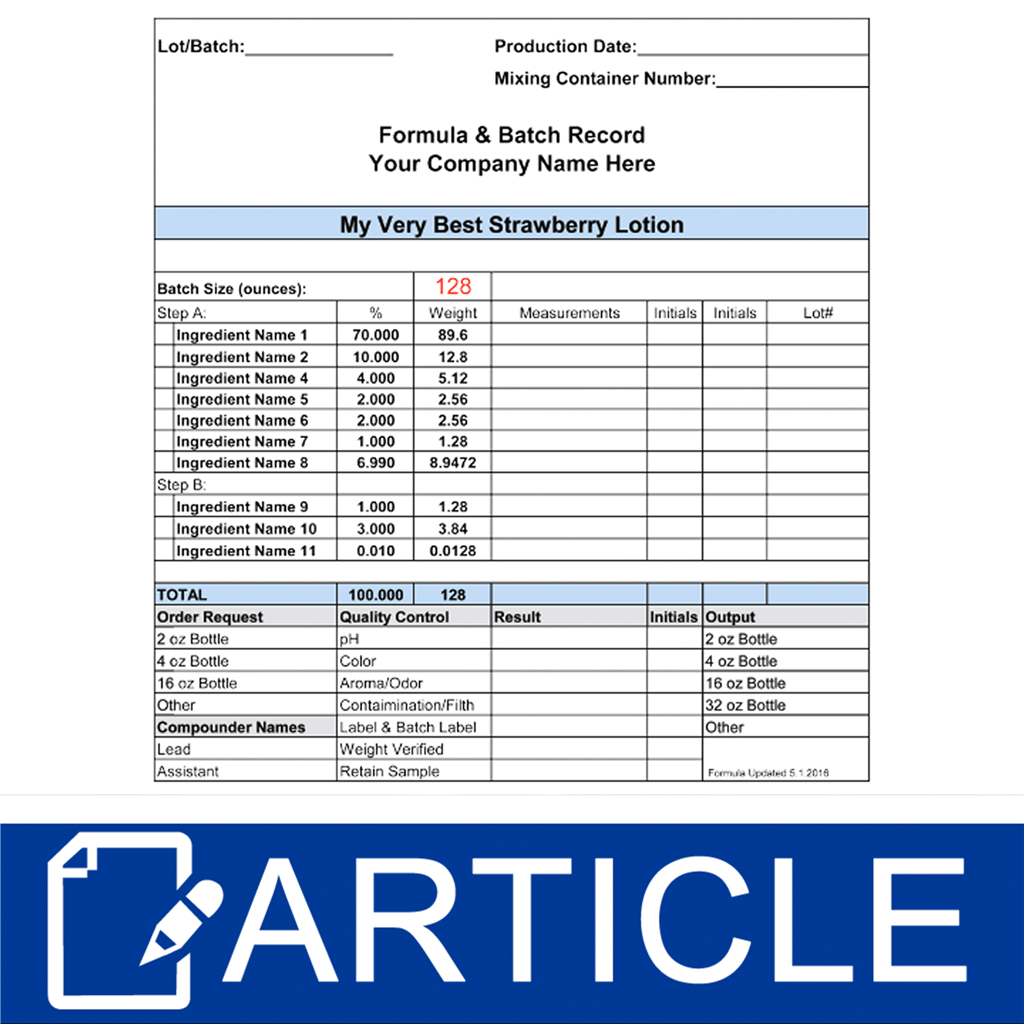
Formula & Batch Records Wholesale Supplies Plus
A batch manufacturing record (BMR) is a comprehensive document that outlines the entire production process for a specific batch of a product. It serves as a detailed guideline for manufacturers to follow in order to ensure consistency and quality control throughout the manufacturing process.

BMR Batch manufacturing record BMR Forms Pharma YouTube
A batch manufacturing record is a written document from the batch that is prepared during the pharmaceutical manufacturing process. It contains step-by-step batch production and actual information of the entire production process. There are several steps in the pharmaceutical product manufacturing process.

Batch Manufacturing Record InstantGMP Pro
The following features can also important for prep batch manufacturing records by the chemical and process manufacturing industry: Step-by-step instructions additionally authentications. BMR software doing a copy a the master formula record, auto-populating instructions how users have lead thru the manufacturing process step by step without.